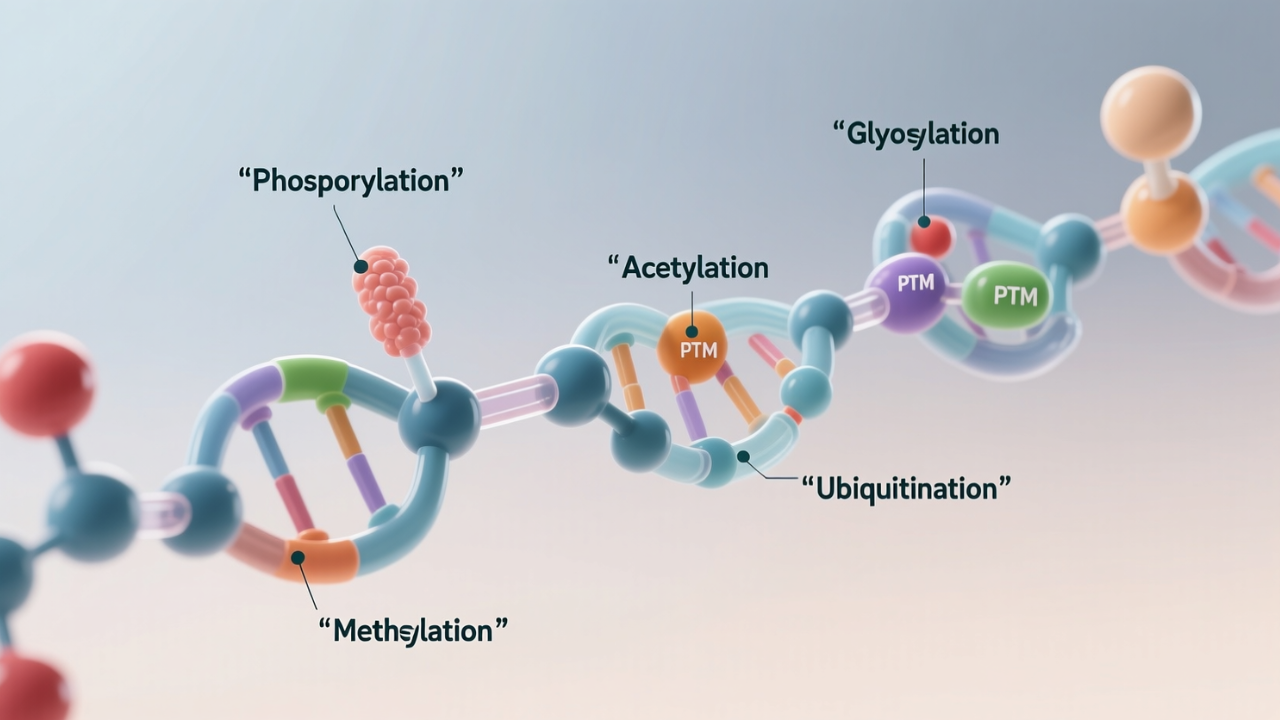
Proteins are not quite functional at the moment when they are formed by ribosomes. Rather, numerous post-translational events occur to the protein, which are often collectively referred to as post-translational modifications (PTMs).
These alterations make a significant impact on the number of functions that proteins can perform, such as control of protein structure stability, activity, location, and interactions with other biomolecules.
PTMs play roles in the processes of signaling, immune response, repair, and programmed cell death.
We will discuss the primary categories of post-translational modifications, their role, and significance in contemporary biomedical studies.
Why Post-Translational Modifications Count?
The proteins are the cellular workhorses that perform structural, enzymatic, and regulatory functions. But it is the fine-tuning, which is the result of PTMs, that makes them specific. In the absence of such changes, numerous proteins would not be able to fold properly and/or localize in the correct compartment and perform the desired activity.
Moreover, the errors of PTMs are connected to cancer, neurodegenerative diseases, metabolic disorders, and autoimmune disorders.
Significant Classes of Post-Translational Processes
01. Phosphorylation
One of the most examined PTMs is phosphorylation. In this process, a phosphate group is added to serine, threonine, or tyrosine. It serves as a molecular switch to activate or inactivate enzymes as well as receptors on one side and controls signaling cascades such as the mitogen-activated protein kinase (MAPK) pathway and insulin signaling on the other side.
Many researchers utilize the good quality of Phospho antibodies online to accurately analyze the pattern of phosphorylation of proteins, which allows tracing the signal pathways and clarifying pathological changes.
02. Ubiquitination
Ubiquitination involves the replacement of ubiquitin, a small protein, with a lysine residue on the intended protein. As the name implies, the effects of ubiquitination are not restricted to degradation.
Although polyubiquitination is commonly used to target proteins to destruction by the proteasome, monoubiquitination may be used to control protein trafficking, DNA repair, and transcription.
03. Acetylation
Depending on the lysine residues, the acetylation is normally vital in the DNA-protein interactions. Chromatin access and gene expression are regulated by histone acetylation, e.g., the balanced acetylation and deacetylation are implicated in such essential processes as stem cell renewal and cellular aging.
HDAC inhibitors, the clinical use of which as drugs is already known in cancer treatment, underline the pathogenic significance of this posttranslational modification.
04. Glycosylation
The addition of sugar groups to proteins by glycosylation is one of the structurally diverse PTMs. Protein folding, stability, immune recognition, and cell-cell communication require it.
The majority of therapeutic antibodies are modified by additional glycosylation as a method of enhancing their stability and efficiency. Malfunctions in glycosylation patterns are associated with inheritable diseases, immune-related diseases, and cancer.
05. Methylation
Methylation on proteins, usually on the residue arginine and lysine residues, is a ubiquitous mechanism of regulating gene expression and protein-protein interactions. Methylation of histone genes plays an essential epigenetic role in determining which set of genes is expressed and which ones remain silent.
06. Lipidation
Lipidation is a covalent modification of proteins by lipid molecules that assists in their anchoring to cell membranes. This change causes the right localization of key signaling proteins.
As an example, Ras GTPases need to be lipid-modified in order to stimulate oncogenic signaling.
07. Proteolytic Cleavage
Certain proteins are formed as inactive precursors (zymogens) whose activation will require cleavage. Proteolytic cleavage occurs to render hormones such as insulin, digestive enzymes, and caspases biologically active. The irreversible alteration plays an important role in the developmental regulation and programmed cell death.
| NOTE: With the addition of specialized reagents, such as a c-Abl antibody, PTMs are essential in exploring the signal transduction pathways. |
Conclusion
Post-translational modifications are not merely additives to proteins; they control the life cycle of cells. Almost all biological processes are mediated by PTMs to refine the activity, stability, and functionality of proteins.
In cancer treatment research or neurodegenerative disease studies, post-translational modifications are the key to the discovery of the next generation of biomedical impact.